Dr. ir. Frans C.H.D. van den Beemt (nuclear physicist)
15 March 2022
The 2021 UN Climate Change Conference, COP 26, resulted in several countries making statements that they would aim to reduce their emissions of greenhouse gases, particularly CO2, based on the fear that these gases are warming the Earth dangerously by what they call the greenhouse effect. But fear is a bad counselor. Understanding the matter reduces anxiety. The purpose of this article is to inform a broad audience about the greenhouse gas CO2 without going into scientific detail, for the latter see [1].
The Earth Thermostat: ensuring a livable temperature on Earth.
The sun heats the Earth with mostly visible light. Our water planet, the Earth, cools down by invisible heat radiation to space. The Earth’s surface, the oceans, and the atmosphere store heat to varying degrees. Wind- and water flows distribute heat all over the Earth. The evaporation process of water transmits energy from the surface into the air. Warm air rises until it cools and forms clouds. Clouds reflect incoming sunlight back to space that otherwise would have warmed the Earth’s surface. Clouds emit heat radiation to space and also some back to the Earth’s surface. These and many more heat transport processes act together as a planet-wide thermostat to create the livable temperature on Earth that we enjoy today.
The terrestrial thermostat knows no rest.
If the Earth’s surface warms up for any reason, this thermostat acts until a new equilibrium is reached. This equilibrium is never static, however, it fluctuates because the wind, convection, precipitation, and all the other dynamic natural processes continually adjust. The atmosphere shows dynamic, cyclic and chaotic characteristics.
What is a greenhouse gas?
Gases that can absorb heat radiation, hold it for a while, and emit it again, are called greenhouse gases, the within our atmosphere most important being CO2 and water vapor.
Energy transport within the atmosphere
Within our atmosphere, two processes are active to transport the energy absorbed by the greenhouse gases:
- First, collisions between air molecules happen all the time. Molecular collisions distribute the energy among the available energy levels between all the molecules involved, this happens within thousandths of a second [3]. Remember that air consists of 78% nitrogen, 20% oxygen, 2% water vapor, and only a mere 0.04% CO2.
- Second, radiation absorption and emission of energy by the greenhouse gases. Spontaneous emissions happen at timescales of the order of 10-3 sec or larger [3]
Greenhouse gaseous molecules that vibrate and rotate, constantly emit radiation. The wavelength of this radiation depends on a number of factors, including the local average temperature, and the properties of the molecules. We can measure the Earth to space emitted radiation by using satellites that look back to the Earth. These satellite data show a wide spectrum of emissions in which we can recognize the emission sources by their specific wavelengths and the effective temperatures of their emissions. Satellites only measure emissions from the atmosphere that on their way to the satellite are not again absorbed. That is why most emissions originate from thin atmospheric layers or from cloud tops.
For water vapor, the emission temperature is around -13 oC, which is a temperature within the troposphere (lower atmosphere) at average cloud altitudes. CO2 emits radiation at about -53 oC, which temperature we find within the stratosphere just above the troposphere. Water vapor shows such a wide absorption spectrum that it overlaps the CO2-specific lines within the troposphere (see fig 1).
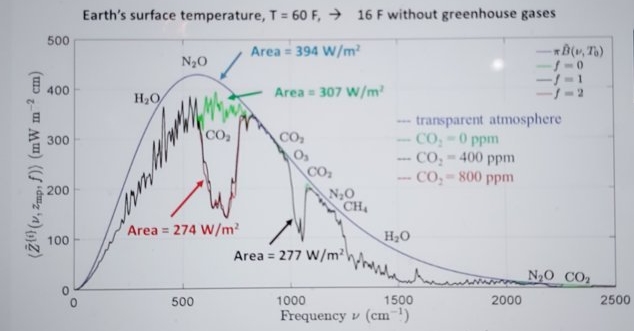
Figure 1 ( copied from Figure 10 in [2]): Effects of changing concentrations of CO2 on the filtered spectral flux at the mesopause altitude of 86 km. The smooth blue line is the spectral flux, from a surface at the temperature of 288.7 K for a transparent atmosphere with no greenhouse gases. The green line is with the CO2 removed but with all the other greenhouse gases at their standard concentrations. The black line is with all greenhouse gases at their standard concentrations. The red line is for twice the standard concentration of CO2 but with all the other greenhouse gases at their standard concentrations. Doubling the standard concentration of CO2 (from 400 to 800 ppm) would only cause a forcing increase (the area between the black and red lines) of 3.0 W m−2.
Above clouds, the water vapor concentration decreases where CO2 concentration stays nearly constant. Within the upper troposphere and the stratosphere, the CO2-specific part of the emission by clouds and water vapor will be again absorbed but now mainly by CO2. Within the thin stratosphere, CO2 emits to space as our satellites detect.
Heat emission to space at top of the atmosphere
The Earth cools to space by heat radiation. But water vapor and CO2 absorb a great part of it.
The absorption spectra for CO2 and water vapor shows such a large overlap that the same amount of heat radiation will be trapped within our lower atmosphere with or without CO2. The difference comes within the thin atmosphere far above our clouds within the stratosphere. Part of the heat radiation emissions from water vapor and from top of clouds is absorbed within the stratosphere by CO2 where water vapor is hardly present. After absorption CO2 emits heat radiation partly into space that cools our Earth. It is clear that without CO2 slightly more heat radiation will be lost to space with the help of water vapor and clouds as the latter emit on average at some higher temperatures.
Line-by-line, spectral studies [2], [3], and [4] find a slight surplus absorbed energy by CO2 doubling. These calculated surplus absorptions happen within the high troposphere and the stratosphere. As such doubling of CO2 potential traps some heat within the high atmosphere. Radiation transport of this surplus heat to lower atmosphere layers will end up in absorption within clouds and water vapor and will be part of further emissions to space. This latter we can regard as indirect emission to space of the by CO2 trapped heat. These theoretical findings need further research but for now, doubling CO2 atmospheric concentration seems not to be a factor that has the potential to warm our Earth’s surface.
Heat emission within the troposphere
In theory, CO2 can retain the additional absorbed heat on average within 10-3 sec or larger. If nothing else happens within a second, CO2 will emit this heat excess again in arbitrary directions.
As long as the atmosphere is dense enough, as it is within the troposphere, the second process (successive absorption and emission) for energy transport is not effective at all. Instead, the first process (collisions between air molecules) is operative. The second process becomes effective within the stratosphere high above the average cloud layers. Within the stratosphere, this is resulting in CO2 emissions to space (including indirect emission) as we are measuring with satellites.
The IPCC suggests that the second process (successive absorption and emission) for energy transport causes CO2 to emit part of the additional absorbed heat radiation back to the earth’s surface, which is thereby heated again. The aim of COP26 is to combat this warming by reducing the CO2 concentration in the atmosphere. But within the lower troposphere, the first process of fast molecular collisions removes all the additional energy from CO2 and no additional energy remains. CO2 is no longer capable to heat the Earth’s surface; this is in line with [2] and [3].
In addition, downward CO2 emission from the stratosphere results in absorption (and successive emission) by water vapor and clouds. Within the high troposphere water vapor and clouds are constantly emitting heat radiation to space, what we call an indirect emission and cooling to space of initial CO2 absorbed radiation.
The trapping and release of radiation by CO2 greenhouse gases
The greenhouse effect stands little chance within the troposphere because the CO2 molecules do not have any excess heat to warm the earth’s surface. The first process for energy transport as mentioned above is effective. The nitrogen, oxygen, and water vapor molecules run off with the heat stolen from CO2 and disappear with the rising air and become part of the Earth’s thermostat as discussed before.
Water vapor and clouds: cooling to space from the upper troposphere
High in the tenuous troposphere, with greatly reduced numbers of molecular collisions, the top of clouds and water vapor radiate heat into space and cool the Earth, as we have observed and measured with satellites.
CO2 cooling to space from the lower stratosphere
The density of atmospheric molecules in the lower stratosphere is sufficiently low that the heat radiations from CO2 can now reach space unimpeded and cool the Earth further. Downward directed CO2 emissions from the cold lower stratosphere support cooling to space indirectly, by first being absorbed in the warmer high troposphere by water vapor and clouds and second by part of emissions to space by water damp and cloud tops as discussed above. As water vapor spectral absorption lines do overlap with CO2 absorption lines hence CO2-specific radiation is absorbed by water vapor.
Frequent asked question #1:
Greenhouse gases are known for their absorption of heat radiation as emitted by the Earth’s surface. Only warm air can rise, hence air close to the surface must warm up first. Then greenhouse gases such as water vapor and CO2 must be the cause of this warming, or not?
Answer #1:
Indeed absorption by greenhouse gases in combination with molecular collisions initially warms the near-surface air at every given moment. But surplus absorbed energy finds no time to accumulate within the lower atmosphere. Part of the surplus absorbed energy will be used to evaporate water that starts rising and the other part will drive the rising of warm air bubbles until a height where cloud forming starts. Where warm air bubbles are rising other cold bubbles will come down and bring a lower temperature near the surface often in conjunction with precipitation. This ongoing energy transport keeps the temperature near-surface in balance and away from warming by greenhouse gases. This process is part of the terrestrial thermostat that needs further research.
Frequent asked question #2:
In the literature, it is mentioned that the calculated average Earth surface temperature without greenhouse gases must be -18oC or 33oC cooler than in the reality of +15oC with greenhouse gases. The greenhouse effect must be real and strong, or not?
Answer #2:
The imaginary temperature ( -18oC) without greenhouse gases (GHG) is calculated with a one-dimensional model where only GHG gases are removed from the calculation and not clouds. Clouds reflect incoming sunlight and are an important factor within this model. Cloud tops have on average about a -13oC temperature in our real atmosphere (with GHG). As such the calculated temperature (-18oC) is not the temperature on the Earth’s surface beneath the cloudy area where it must be warmer. Clouds are a direct result of having water vapor (a strong GHG gas) within our atmosphere. As such also clouds have to be removed when we remove GHG in our model. The calculation of the temperature at the surface without clouds and without GHG results in +2 oC [1] (and compare this with +5 oC [2] but now also without ice caps and without oceans). Hence, it is not -18 oC as in most scientific literature. Thus the Earth without GHG is not 33 oC but only 13oC cooler than the reality of +15oC with GHG. It remains to be seen whether this mild temperature difference can be completely attributed to clouds as part of the terrestrial thermostat. Because of its complexity, further research on this thermostat is recommended.
Frequent asked question #3:
Satellites measure substantive absorption by CO2 that would not be there without CO2 in our atmosphere. As such CO2 does warm the Earth, or not?
Answer #3:
Absorption by CO2 is not the same as limiting Earth’s radiation to space and consequently warming the Earth. First, there is a difference between direct and indirect limiting of Earth heat radiation to space and direct or indirect warming of the Earth. And warming the Earth as we measure it, means often warming the air layer at 1,5m above the surface or the surface itself.
Firstly, satellites measure indeed less radiation to space within the CO2-specific spectra lines from the low stratosphere (about above 10km). But CO2 back radiation (downwards instead of to space) will be absorbed both by the top of clouds and the water vapor within the upper troposphere. The indirect cooling radiation to space then comes from these clouds and water vapor as an emission within frequencies for a large part outside that of the CO2 band. Or in other words via back radiation, the initial CO2– absorbed energy ultimately ends up in space indirectly and still cools the Earth.
Secondly, any absorption by CO2 within the lower atmosphere near-surface (first 100 meters) will not per definition warms the air at 1,5m above Earth’s surface. Because that absorbed heat will first be used both to evaporate more water into water vapor and will be used to rise warm air bubbles that immediately will be replaced with cold air bubbles. Cold air bubbles can arrive from clouds together with precipitation that cools the air on their way downwards or can arrive from colder places by wind and convection.
Conclusion
The greenhouse effect of CO2, as described by IPCC and others, does not stand a chance of success within our dense lower atmosphere. Close to the Earth’s surface, during molecular collisions, CO2 is forced to relinquish immediately the absorbed additional heat to its environment. Rising air then transports that heat to the high, thin atmosphere. The greenhouse effect of CO2 is nipped in the bud and has no chance. There is no need to be afraid of CO2. Instead, there is a need to gain more knowledge about our water thermostat.
Literature:
[1] This study of Dr. Ir. Frans C.H.D. van den Beemt (nuclear physicist) is after scientific review first published within the N! magazine begin spring 2021, title: “The warming effect of clouds” https://veni.nl/images/N/N43.pdf page 34 and 35 under Science.
Next it has been extended and published at the website of the international organization Clintel again after an international scientific review, end of spring 2021, title: “The warming effect of clouds” https://clintel.org/the-warming-effect-of-clouds/
At the beginning of summer 2021, a strongly abridged version for laymen in Dutch was published in the neighborhood magazine De Blaak in Tilburg, volume 42 no. 249 June 2021 page 28, title: “Broeikaswerking van CO2” https://deblaak.nl/wp-content/uploads/2021/08/nummer-249-site.pdf
[2] THE ROLE OF GREENHOUSE GASES Jan 30, 2022, W. A. van Wijngaarden and W. Happer Respectively Department of Physics and Astronomy, York University, Canada and Department of Physics, Princeton University, USA https://co2coalition.org/publications/infrared-forcing-by-greenhouse-gases
[3] The absorption of thermal emitted infrared radiation by CO2, Published on April 3, 2020, Principia Scientific Intl. , W.J. Witteman, Emeritus professor University of Twente (NL) https://principia-scientific.org/the-absorption-of-thermal-emitted-infrared-radiation-by-co2/
Revised February 2022: Global warming by thermal absorption of CO2 February 2022 http://www.clepair.net/witteman-CO2+IR.html
[4] The physics of doubling CO2 (full version) Frans van den Beemt https://sciencetalks.nl/the-physics-of-doubling-co2-full-version/

Hi Frans
I fully agree with you that “we” should not be feared about greenhouse gasses. Fortunately they exist. I like you comprehensive description and analysis of the “thermostat” of the Earth.
You know I have strong objection against back radiation model of Co2, meaning the absorbed IR radiation within the greenhouse gasses (partly coming from the surface), is back radiated to the planet surface and therefore heating subsequently the surface (instead of slowing down the cooling due to the opacity of the air for IR). Is this not “IPCC trilemma” in analogy of Munchhausen’s one??
You stated that the exchange of heat via collisions is the main route from absorbed IR to heating the atmosphere. You addressed the issue in “Answer #1” with the model of rising heated bubbles and falling cold bubbles (nice model). You wrote the following: ” This ongoing energy transport keeps the temperature near-surface in balance and away from warming by greenhouse gases. This process is part of the terrestrial thermostat that needs further research.”
Frans I am playing now the devil’s advocate:
It is hideous complex to estimate if this upward and downward energy transport results in a net growth of heat (temperature) in a world with increasing Co2, because one cannot estimate the change in energy from day to day. The insulation is varying from day to day dependent on day of the year and latitude, the relative humidity (and so transport of heat via water cycle, a very crucial heat transport), the horizontal transport of cold and warm air, dependency of surface texture, etc. In this way you end with the Navier Stokes equations again, and so no answer because you cannot solve these equations. (do not mention climate models!)
Please can you elaborate on “which further research” is required avoiding this NS problem.
Dear Peter van Toorn, Thank you for your comments.
An increasing CO2 concentration absorbs the same IR radiation in the near surface region due to full saturation above 200ppm. I have used a one-dimensional model to focus on the first principles. I agree that the said “near surface temperature balance” will be part of a complex system. This complex system requires further investigation. Within climate models, the Navier Stokes equations are a serious bottleneck and all kinds of correction factors are used instead. No wonder IPCC mentions 38 different climate models. Further research is needed on clouds and ocean currents as the Atlantic Multi-decadal Oscillation (AMO) and the Atlantic Multi-decadal Oscillation (AMO) and much more.
Mission impossible; see:
https://www.clepair.net/complexity.html
Apart from that it is a valuable counter argument on all the nonsense published by other scientists and their echos, who engage in doing the impossible, while only spreading fear.
Fossile fuels are not the problem.
The real causes of
— the rise of the temperature-measurements
— the rise of the CO2-concentration in the air
— the threat to the permafrost in particular in Siberia
are peristently not being researched.
It is easy to understand that the fossile fuels do not cause the rise of the CO2-concentration.
For those who can read German language; uumwelt de (point before de)(with uu)
More economic Windpower is possible, see turkmobile com
best regards, vd Veen